Chlorine Has Electron Gain Enthalpy . The electron gain enthalpy of elements becomes more negative as we move from left to right in a period. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added to the atom. The electron gain enthalpy of an atom is negative when energy is released on the addition of an electron. In the case of chlorine and fluorine, chlorine has a higher negative electron gain enthalpy value. Among fluorine and chlorine, chlorine has a higher negative electron gain enthalpy value. Exception in electron gain enthalpy: The electron being gained by fluorine would be taken in to a much smaller 2p orbital and requires more electron coupling. It is due to small size of fluorine atom. Chlorine has the most negative electron gain enthalpy. Variation of electron gain enthalpy along a period. It can be used to estimate the stability. The negative electron gain enthalpy of fluorine is less than that of chlorine.
from askfilo.com
It is due to small size of fluorine atom. It can be used to estimate the stability. In the case of chlorine and fluorine, chlorine has a higher negative electron gain enthalpy value. The electron gain enthalpy of an atom is negative when energy is released on the addition of an electron. The electron being gained by fluorine would be taken in to a much smaller 2p orbital and requires more electron coupling. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added to the atom. Exception in electron gain enthalpy: Chlorine has the most negative electron gain enthalpy. Among fluorine and chlorine, chlorine has a higher negative electron gain enthalpy value. The electron gain enthalpy of elements becomes more negative as we move from left to right in a period.
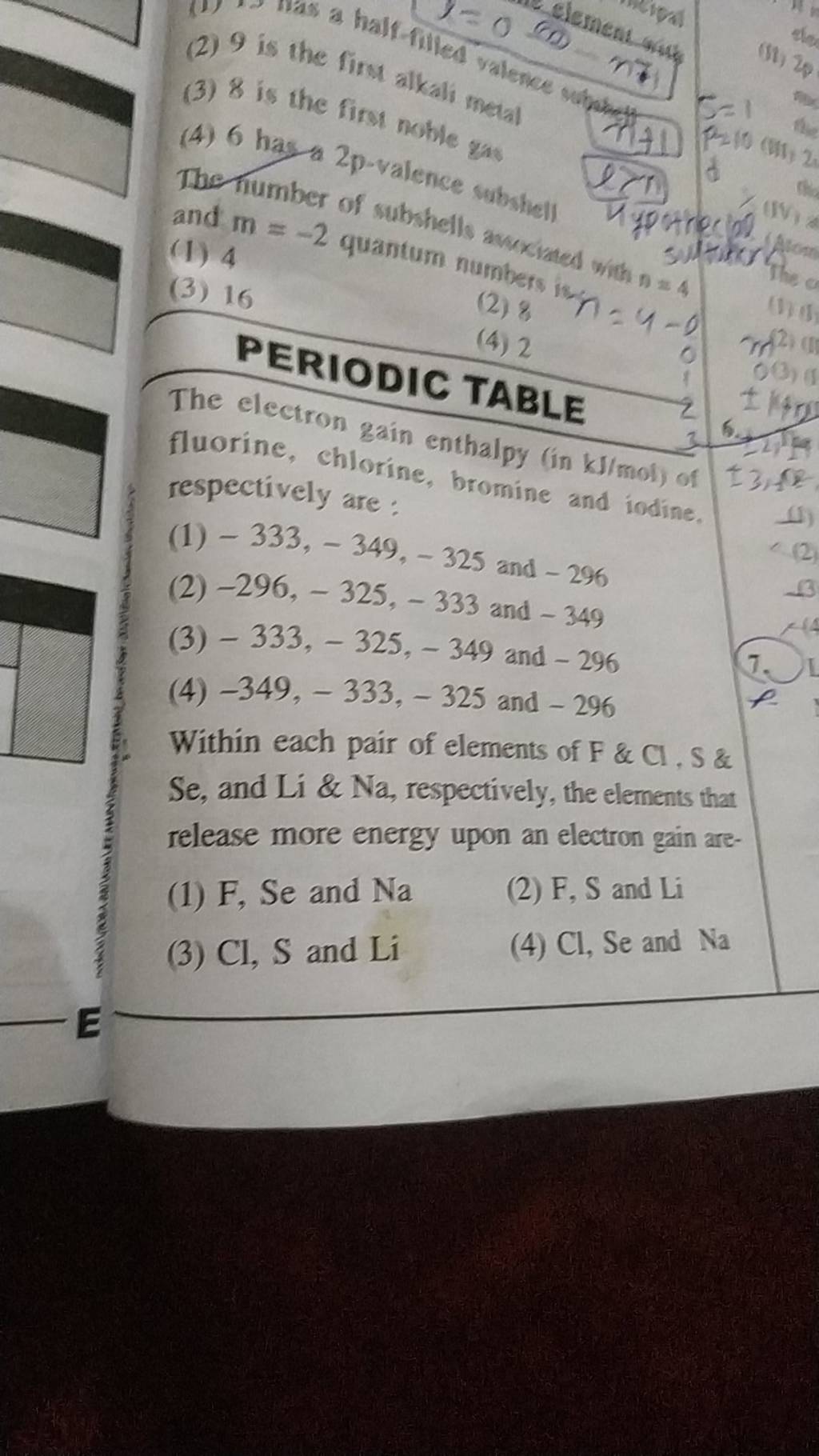
PERIODIC TABLE The electron gain enthalpy (in kJ/mol ) of fluorine, chlor..
Chlorine Has Electron Gain Enthalpy In the case of chlorine and fluorine, chlorine has a higher negative electron gain enthalpy value. In the case of chlorine and fluorine, chlorine has a higher negative electron gain enthalpy value. The electron being gained by fluorine would be taken in to a much smaller 2p orbital and requires more electron coupling. Chlorine has the most negative electron gain enthalpy. Exception in electron gain enthalpy: The electron gain enthalpy of an atom is negative when energy is released on the addition of an electron. It can be used to estimate the stability. It is due to small size of fluorine atom. Variation of electron gain enthalpy along a period. The electron gain enthalpy of elements becomes more negative as we move from left to right in a period. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added to the atom. The negative electron gain enthalpy of fluorine is less than that of chlorine. Among fluorine and chlorine, chlorine has a higher negative electron gain enthalpy value.
From www.nagwa.com
Question Video Identifying the Diagram Representing How Chlorine Chlorine Has Electron Gain Enthalpy Chlorine has the most negative electron gain enthalpy. Variation of electron gain enthalpy along a period. In the case of chlorine and fluorine, chlorine has a higher negative electron gain enthalpy value. It is due to small size of fluorine atom. It can be used to estimate the stability. Among fluorine and chlorine, chlorine has a higher negative electron gain. Chlorine Has Electron Gain Enthalpy.
From byjus.com
Compare the electron gain enthalpy of 2nd period. Chlorine Has Electron Gain Enthalpy Among fluorine and chlorine, chlorine has a higher negative electron gain enthalpy value. The electron gain enthalpy of an atom is negative when energy is released on the addition of an electron. Exception in electron gain enthalpy: It can be used to estimate the stability. Chlorine has the most negative electron gain enthalpy. In the case of chlorine and fluorine,. Chlorine Has Electron Gain Enthalpy.
From www.meritnation.com
pls explain why fluorine has less negative electron gain enthalpy than Chlorine Has Electron Gain Enthalpy The negative electron gain enthalpy of fluorine is less than that of chlorine. In the case of chlorine and fluorine, chlorine has a higher negative electron gain enthalpy value. Chlorine has the most negative electron gain enthalpy. Among fluorine and chlorine, chlorine has a higher negative electron gain enthalpy value. The electron being gained by fluorine would be taken in. Chlorine Has Electron Gain Enthalpy.
From www.doubtnut.com
Flourine has more negative electron gain enthalpy than chlorine.(T/F) Chlorine Has Electron Gain Enthalpy Among fluorine and chlorine, chlorine has a higher negative electron gain enthalpy value. Variation of electron gain enthalpy along a period. The negative electron gain enthalpy of fluorine is less than that of chlorine. Chlorine has the most negative electron gain enthalpy. The electron gain enthalpy of elements becomes more negative as we move from left to right in a. Chlorine Has Electron Gain Enthalpy.
From askfilo.com
(i) The element chlorine (Cl) has the highest negative electron gain enth.. Chlorine Has Electron Gain Enthalpy The electron gain enthalpy of elements becomes more negative as we move from left to right in a period. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added to the atom. Chlorine has the most negative electron gain enthalpy. The electron being gained by fluorine. Chlorine Has Electron Gain Enthalpy.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Chlorine Has Electron Gain Enthalpy The electron gain enthalpy of an atom is negative when energy is released on the addition of an electron. It is due to small size of fluorine atom. The electron gain enthalpy of elements becomes more negative as we move from left to right in a period. It can be used to estimate the stability. The electron being gained by. Chlorine Has Electron Gain Enthalpy.
From byjus.com
6. d the electron affinity of chlorine from the following data Chlorine Has Electron Gain Enthalpy Variation of electron gain enthalpy along a period. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added to the atom. It is due to small size of fluorine atom. It can be used to estimate the stability. The electron gain enthalpy of elements becomes more. Chlorine Has Electron Gain Enthalpy.
From www.toppr.com
Why is the electron gain enthalpy of chlorine more negative than fluorine? Chlorine Has Electron Gain Enthalpy In the case of chlorine and fluorine, chlorine has a higher negative electron gain enthalpy value. It is due to small size of fluorine atom. Chlorine has the most negative electron gain enthalpy. Among fluorine and chlorine, chlorine has a higher negative electron gain enthalpy value. The electron gain enthalpy of elements becomes more negative as we move from left. Chlorine Has Electron Gain Enthalpy.
From www.nagwa.com
Question Video Recalling the Species Formed When a Chlorine Atom Gains Chlorine Has Electron Gain Enthalpy In the case of chlorine and fluorine, chlorine has a higher negative electron gain enthalpy value. The electron gain enthalpy of an atom is negative when energy is released on the addition of an electron. Among fluorine and chlorine, chlorine has a higher negative electron gain enthalpy value. It is due to small size of fluorine atom. The negative electron. Chlorine Has Electron Gain Enthalpy.
From brainly.in
why is electron gain enthalpy of chlorine greater than flourine Chlorine Has Electron Gain Enthalpy The electron being gained by fluorine would be taken in to a much smaller 2p orbital and requires more electron coupling. Among fluorine and chlorine, chlorine has a higher negative electron gain enthalpy value. The negative electron gain enthalpy of fluorine is less than that of chlorine. In the case of chlorine and fluorine, chlorine has a higher negative electron. Chlorine Has Electron Gain Enthalpy.
From www.numerade.com
SOLVEDWhich process changes a chlorine atom into a chloride ion? A Chlorine Has Electron Gain Enthalpy The negative electron gain enthalpy of fluorine is less than that of chlorine. The electron gain enthalpy of an atom is negative when energy is released on the addition of an electron. Chlorine has the most negative electron gain enthalpy. The electron being gained by fluorine would be taken in to a much smaller 2p orbital and requires more electron. Chlorine Has Electron Gain Enthalpy.
From www.youtube.com
electron gain enthalpy class 11 Electron gain enthalpy of fluorine is Chlorine Has Electron Gain Enthalpy It is due to small size of fluorine atom. Chlorine has the most negative electron gain enthalpy. In the case of chlorine and fluorine, chlorine has a higher negative electron gain enthalpy value. The negative electron gain enthalpy of fluorine is less than that of chlorine. Among fluorine and chlorine, chlorine has a higher negative electron gain enthalpy value. The. Chlorine Has Electron Gain Enthalpy.
From brainly.in
The electron gain enthalpy of chlorine is 349 kJ mol1. How much Chlorine Has Electron Gain Enthalpy It can be used to estimate the stability. In the case of chlorine and fluorine, chlorine has a higher negative electron gain enthalpy value. The electron gain enthalpy of elements becomes more negative as we move from left to right in a period. Chlorine has the most negative electron gain enthalpy. Variation of electron gain enthalpy along a period. Among. Chlorine Has Electron Gain Enthalpy.
From www.doubtnut.com
The electron gain enthalpy ( in kJ//mol ) of fluorine, chlorine, Chlorine Has Electron Gain Enthalpy The electron gain enthalpy of an atom is negative when energy is released on the addition of an electron. Exception in electron gain enthalpy: The negative electron gain enthalpy of fluorine is less than that of chlorine. It can be used to estimate the stability. Variation of electron gain enthalpy along a period. The electron being gained by fluorine would. Chlorine Has Electron Gain Enthalpy.
From byjus.com
What is difference between ionization enthalpy and electron gain Chlorine Has Electron Gain Enthalpy Among fluorine and chlorine, chlorine has a higher negative electron gain enthalpy value. The electron gain enthalpy of an atom is negative when energy is released on the addition of an electron. It can be used to estimate the stability. Variation of electron gain enthalpy along a period. The negative electron gain enthalpy of fluorine is less than that of. Chlorine Has Electron Gain Enthalpy.
From www.doubtnut.com
[Punjabi] Explain Electron gain enthalpy of chlorine is more negative Chlorine Has Electron Gain Enthalpy It can be used to estimate the stability. Chlorine has the most negative electron gain enthalpy. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added to the atom. Variation of electron gain enthalpy along a period. In the case of chlorine and fluorine, chlorine has. Chlorine Has Electron Gain Enthalpy.
From www.toppr.com
The electron gain enthalpy of chlorine is 3.7eV . How much energy in Chlorine Has Electron Gain Enthalpy The electron being gained by fluorine would be taken in to a much smaller 2p orbital and requires more electron coupling. It is due to small size of fluorine atom. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added to the atom. In the case. Chlorine Has Electron Gain Enthalpy.
From www.youtube.com
The electron gain enthalpy (in \( \mathrm{kJ} / \mathrm{mol} \) ) of Chlorine Has Electron Gain Enthalpy The electron being gained by fluorine would be taken in to a much smaller 2p orbital and requires more electron coupling. The negative electron gain enthalpy of fluorine is less than that of chlorine. Variation of electron gain enthalpy along a period. The electron gain enthalpy of elements becomes more negative as we move from left to right in a. Chlorine Has Electron Gain Enthalpy.